
Chemistry, 08.11.2019 23:31 astultz309459
Suppose 200.0 ml of 1.00 m hcl and 200.0 ml of 1.00 m naoh, both initially at 21.0°c, are mixed in a thermos flask. when the reaction is complete, the temperature is 27.8°c. assuming that the solutions have the same heat capacity as pure water, compute the heat released (in kj).

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Arock can be broken down into different kinds of substances by physical processes. no chemical reactions are needed to separate different parts of a rock into pure substances. this is because a rock is a(n)
Answers: 1

Chemistry, 22.06.2019 05:20
Identify and describe the three ways that mutations affect organisms.
Answers: 1

Chemistry, 22.06.2019 20:00
There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. in the first step, calcium carbide and water react to form acetylene and calcium hydroxide: cac2 (s) + 2h2o (g) → c2h2 (g) + caoh2 (s) =δh−414.kj in the second step, acetylene, carbon dioxide and water react to form acrylic acid: 6c2h2 (g) + 3co2 (g) + 4h2o (g) → 5ch2chco2h (g) =δh132.kj calculate the net change in enthalpy for the formation of one mole of acrylic acid from calcium carbide, water and carbon dioxide from these reactions. round your answer to the nearest kj .
Answers: 3

Chemistry, 23.06.2019 00:30
In a ball-and-stick molecular model, what do the sticks represent?
Answers: 1
You know the right answer?
Suppose 200.0 ml of 1.00 m hcl and 200.0 ml of 1.00 m naoh, both initially at 21.0°c, are mixed in a...
Questions


Social Studies, 17.02.2021 18:30


Mathematics, 17.02.2021 18:30

Biology, 17.02.2021 18:30


English, 17.02.2021 18:30

SAT, 17.02.2021 18:30

History, 17.02.2021 18:40


Mathematics, 17.02.2021 18:40


Advanced Placement (AP), 17.02.2021 18:40

Mathematics, 17.02.2021 18:40

Mathematics, 17.02.2021 18:40


Chemistry, 17.02.2021 18:40

History, 17.02.2021 18:40


Mathematics, 17.02.2021 18:40


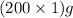 = 200 g
= 200 g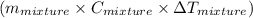
 represents change in temperature
represents change in temperature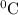 )
)![[400g\times 4.186J.g^{-1}.^{0}\textrm{C}^{-1}\times(27.8-21.0)^{0}\textrm{C} ]](/tpl/images/0366/2490/9c7ad.png) =
= 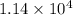 J=11.4 kJ
J=11.4 kJ


