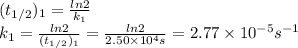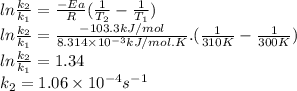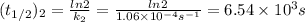For the reaction:
2n2o5(g) → 4no2(g) + o2(g) the rate law is: (δ[o2]/δt) = k[n2o5] at 300 k...

Chemistry, 08.11.2019 22:31 alexciamartinez05
For the reaction:
2n2o5(g) → 4no2(g) + o2(g) the rate law is: (δ[o2]/δt) = k[n2o5] at 300 k, the half-life is 2.50 × 104 seconds and the activation energy is 103.3 kj/mol. what is the half-life at 310 k? (hint: use rate law expression to determine the reaction order → solve for k1 at 300 k using the corresponding half-life expression → use two-point arrhenius equation to solve for k2 at 310 k → use the half-life expression again to solve for half-life at 310 k)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:30
Which of the following has wavelength longer than the wavelength of viable light? a) x rays b) gamma rays c) radios waves d) ultraviolet waves
Answers: 1

Chemistry, 22.06.2019 18:00
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2

Chemistry, 23.06.2019 00:30
Element j is 1s 2s 2p 3s . (i) how many unpaired electrons does j have? (ii) is j a good oxidizing agent or a reducing agent? (iii) state reason for the answer.
Answers: 1

Chemistry, 23.06.2019 05:40
Convert a speed of 201 cm/s to units of inches per minute. also, show the unit analysis by dragging components into the unit‑factor slots.
Answers: 1
You know the right answer?
Questions



Mathematics, 22.04.2020 19:59

Social Studies, 22.04.2020 19:59

Mathematics, 22.04.2020 19:59

Mathematics, 22.04.2020 19:59

Mathematics, 22.04.2020 19:59




Chemistry, 22.04.2020 19:59


Mathematics, 22.04.2020 19:59

Geography, 22.04.2020 19:59


Mathematics, 22.04.2020 19:59



Mathematics, 22.04.2020 19:59

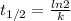
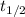 is the half-life
is the half-life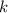 is the rate constant
is the rate constant