
Chemistry, 08.11.2019 06:31 kaziyahf2006
Amixture of methane gas, ch4(g) , and pentane gas, c5h12(g) , has a pressure of 0.5799 atm when placed in a sealed container. the complete combustion of the mixture to carbon dioxide gas, co2(g) , and water vapor, h2o(g) , was achieved by adding exactly enough oxygen gas, o2(g) , to the container. the pressure of the product mixture in the sealed container is 2.378 atm . calculate the mole fraction of methane in the initial mixture, assuming the temperature and volume remain constant.

Answers: 1


Another question on Chemistry


Chemistry, 21.06.2019 22:30
Explain why scientists use shared characteristics to make cladograms.
Answers: 1

Chemistry, 22.06.2019 00:10
According to the diagram; a) identify the anode of the cell and write the half-reaction that occurs there b) write the overall equation for the reaction that occurs as the cell operates c) calculate the value of the standard cell potential ,e cell. d)write the shorthand notation of the cell above e)indicate the flow of the electrons on the diagram
Answers: 3

Chemistry, 22.06.2019 06:30
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1
You know the right answer?
Amixture of methane gas, ch4(g) , and pentane gas, c5h12(g) , has a pressure of 0.5799 atm when plac...
Questions


English, 23.01.2022 21:30

Mathematics, 23.01.2022 21:30

Mathematics, 23.01.2022 21:30

Mathematics, 23.01.2022 21:30

English, 23.01.2022 21:30




History, 23.01.2022 21:30

Mathematics, 23.01.2022 21:30



Mathematics, 23.01.2022 21:30


Mathematics, 23.01.2022 21:30


Biology, 23.01.2022 21:40


English, 23.01.2022 21:40

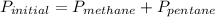
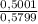 = 0,8624
= 0,8624

