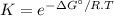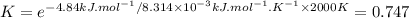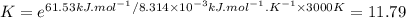Chemistry, 08.11.2019 05:31 ekerns2000paa19x
The decomposition of a generic diatomic element in its standard state is represented by the equation 12x2(g)⟶x(g) assume that the standard molar gibbs energy of formation of x(g) is 4.84 kj·mol−1 at 2000 . k and −61.53 kj·mol−1 at 3000 . k. determine the value of the thermodynamic equilibrium constant, k , at each temperature. at 2000 . k, δ=4.84 kj·mol−1 . what is k at that temperature?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:40
Which statement is usually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 3

Chemistry, 22.06.2019 12:30
Which element has the lowest electronegativity? calcium(ca) gallium(ga) selenium(se) bromine(br)
Answers: 1

Chemistry, 22.06.2019 15:30
Draw the lewis dot structure for each of the following polyatomic ions
Answers: 1

You know the right answer?
The decomposition of a generic diatomic element in its standard state is represented by the equation...
Questions





Geography, 12.12.2019 01:31

Geography, 12.12.2019 01:31

Biology, 12.12.2019 01:31


Health, 12.12.2019 01:31


Social Studies, 12.12.2019 01:31

Advanced Placement (AP), 12.12.2019 01:31

Geography, 12.12.2019 01:31

English, 12.12.2019 01:31



Mathematics, 12.12.2019 01:31



Social Studies, 12.12.2019 01:31