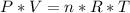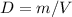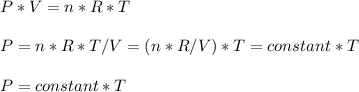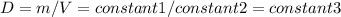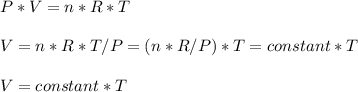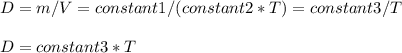Chemistry, 08.11.2019 03:31 kamkam5791
Consider two different containers, each filled with of . one of the containers is rigid and has constant volume. the other container is flexible (like a balloon) and is capable of changing its volume to keep the external pressure and internal pressure equal to each other. if you raise the temperature in both containers, what happens to the pressure and density of the gas inside each container? assume a constant external pressure.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 09:00
What term is missing from the central region that describes hypotheses, theories, and laws? popular predictable mathematical falsifiable
Answers: 2

Chemistry, 22.06.2019 15:00
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1

You know the right answer?
Consider two different containers, each filled with of . one of the containers is rigid and has cons...
Questions


Mathematics, 24.03.2020 21:51


Mathematics, 24.03.2020 21:51





Physics, 24.03.2020 21:51






Computers and Technology, 24.03.2020 21:52

Chemistry, 24.03.2020 21:52

Biology, 24.03.2020 21:52


Biology, 24.03.2020 21:52