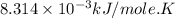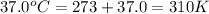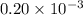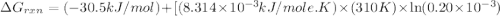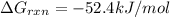Chemistry, 07.11.2019 22:31 brandon56238
Acritical reaction in the production of energy to do work or drive chemical reactions in biological systems is the hydrolysis of adenosine triphosphate, atp, to adenosine diphosphate, adp, as described by the reaction atp(aq)+h2o(l)⟶adp(aq)+hpo2−4(aq) for which δ∘rxn=−30.5 kj/mol at 37.0 °c and ph 7.0. calculate the value of δ in a biological cell in which [atp]=5.0 mm, [adp]=0.20 mm, and [hpo2−4]=5.0 mm.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Transportation is the largest single source of air pollution in the united states. air pollution can harm the environment and human health. which technology could offer a solution to this problem? mufflers that reduce noise motors that run on electricity tires that improve gas mileage
Answers: 3

Chemistry, 22.06.2019 19:00
Which change to the system wood cause the freely-moving piston to lower?
Answers: 1

Chemistry, 22.06.2019 20:30
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1

You know the right answer?
Acritical reaction in the production of energy to do work or drive chemical reactions in biological...
Questions

Mathematics, 31.01.2020 20:56

Physics, 31.01.2020 20:56


History, 31.01.2020 20:56

History, 31.01.2020 20:56

Mathematics, 31.01.2020 20:56

Geography, 31.01.2020 20:56







Mathematics, 31.01.2020 20:56


Health, 31.01.2020 20:56




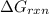 is -52.4 kJ/mol
is -52.4 kJ/mol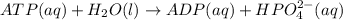
![Q=\frac{[ADP][HPO_4^{2-}]}{[ATP]}](/tpl/images/0364/4024/ccdf0.png)
![[ATP]](/tpl/images/0364/4024/bda18.png) = 5.0 mM
= 5.0 mM![[ADP]](/tpl/images/0364/4024/68360.png) = 0.20 mM
= 0.20 mM![[HPO_4^{2-}]](/tpl/images/0364/4024/c0ca9.png) = 5.0 mM
= 5.0 mM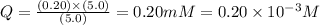
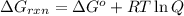 ............(1)
............(1)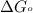 = standard Gibbs free energy = -30.5 kJ/mol
= standard Gibbs free energy = -30.5 kJ/mol