Chemistry, 07.11.2019 22:31 jerrica988
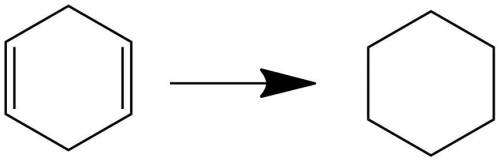
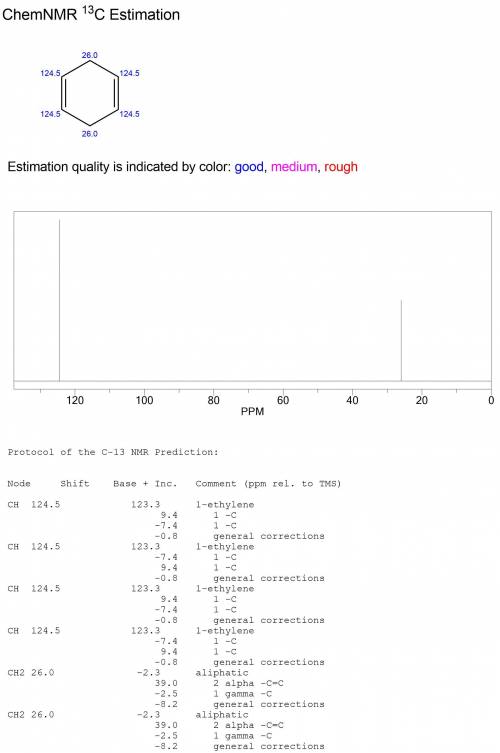
Compound o (c6h8) reacts with two molar equivalents of hydrogen in the presence of a catalyst to produce p (c6h12). the proton-decoupled 13c spectrum of o consists of two singlets, one at δ 26.0 and one at δ 124.5. in the dept 13c spectrum of o the signal at δ 26.0 appears as a ch2 group and the one at δ 124.5 appears as a ch group. note: all structures should be drawn with no bonds between carbon and hydrogen. draw the correct structure for o and p

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Based on the law of conservation of energy, which statement is false? answer- energy is lost when machines dont work right
Answers: 1



Chemistry, 22.06.2019 14:50
Consider the following multistep reaction: a b→ab(slow) a ab→a2b(fast)−−−−−−−−−−−−−−−−− 2a b→a2b(overall) based on this mechanism, determine the rate law for the overall reaction. express your answer in standard masteringchemistry format. for example, if the rate law is k[a]3[b]2 type k*[a]^3*[b]^2
Answers: 3
You know the right answer?
Compound o (c6h8) reacts with two molar equivalents of hydrogen in the presence of a catalyst to pro...
Questions


Computers and Technology, 23.08.2019 16:30

Mathematics, 23.08.2019 16:30





Physics, 23.08.2019 16:30








Mathematics, 23.08.2019 16:30

Mathematics, 23.08.2019 16:30



Mathematics, 23.08.2019 16:30