Chemistry, 07.11.2019 21:31 BigGirlsTheBest
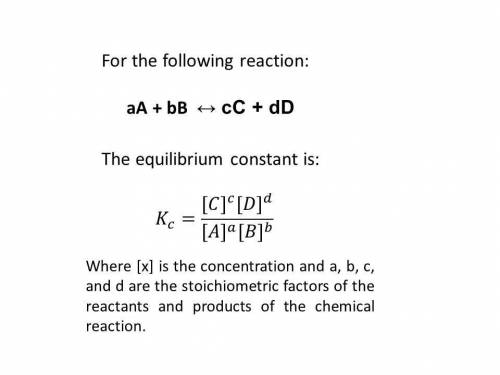
At a certain temperature, the equilibrium constant, kc, kc, for this reaction is 53.3. h2(g)+i2(g)↽−−⇀2hi(g)kc=53.3 h2(g)+i2(g)↽−−⇀2hi(g)kc=53.3 at this temperature, 0.400 mol h20.400 mol h2 and 0.400 mol i20.400 mol i2 were placed in a 1.00 l container to react. what concentration of hihi is present at equilibrium?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
50 pts plz what is the physical state of matter of baking soda.
Answers: 1

Chemistry, 22.06.2019 11:30
Compare and contrast refraction of light and sound will give brainliest
Answers: 1


You know the right answer?
At a certain temperature, the equilibrium constant, kc, kc, for this reaction is 53.3. h2(g)+i2(g)↽−...
Questions

History, 16.04.2021 04:00





Mathematics, 16.04.2021 04:00




History, 16.04.2021 04:00




Mathematics, 16.04.2021 04:00

Biology, 16.04.2021 04:00

Physics, 16.04.2021 04:00

Mathematics, 16.04.2021 04:00


Advanced Placement (AP), 16.04.2021 04:00

![K_{c} = \frac{[HI]^{2}}{[H_{2}][I_{2}]} = 53.3](/tpl/images/0364/2800/9c8ef.png) (2)
(2)![[H_{2}] = [I_{2}] = \frac{0.400 mol}{1 L} = 0.400 mol/L](/tpl/images/0364/2800/3a013.png)
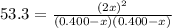
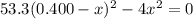
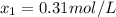
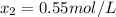
![[HI] = 2x = 2*0.31 mol/L = 0.62 mol/L](/tpl/images/0364/2800/ce8eb.png)
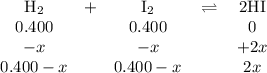
![K_{\text{c}} = \dfrac{\text{[HI]$^{2}$}}{\text{[H$_{2}$][I$_2$]}} = \dfrac{(2x)^{2}}{(0.400 - x)^{2}} = 53.3\\\\\begin{array}{rcl}\dfrac{(2x)^{2}}{(0.400 - x)^{2}} &=& 53.3\\ \dfrac{2x }{0.400 - x} & = & 7.301\\\\2x & = & 7.301(0.400 - x)\\2x & = & 2.920 - 7.301x\\9.301x & = & 2.920\\x & = & \dfrac{2.920}{9.301}\\\\x & = & \mathbf{0.3140}\\\end{array}](/tpl/images/0364/2800/cfef9.png)