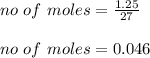Assuming you start with 1.25 g of pure aluminum, calculate the following:
1) the amount of po...


Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
You are to give ampicillin with a recommended dose of 25mg/kg to a child with a mass of 29kg. if stock on hand is 250mg/capsule how many capsules should be given?
Answers: 1


Chemistry, 22.06.2019 10:00
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1

Chemistry, 22.06.2019 20:10
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2
You know the right answer?
Questions

Biology, 05.05.2020 13:14





Physics, 05.05.2020 13:14

History, 05.05.2020 13:14


Mathematics, 05.05.2020 13:14

Mathematics, 05.05.2020 13:14

Mathematics, 05.05.2020 13:14

English, 05.05.2020 13:14

Mathematics, 05.05.2020 13:14


History, 05.05.2020 13:14

Social Studies, 05.05.2020 13:14


French, 05.05.2020 13:14

English, 05.05.2020 13:14