
Chemistry, 07.11.2019 06:31 danielobanoyen
Initially 2.0 moles of n2(g) and 4.0 moles of h2(g) were added to a 1.0-liter container and the following reaction then occurred: 3h2(g) + n2(g) 2nh3(g) the equilibrium concentration of nh3(g) = 0.55 moles/liter at 700.°c. what is the value for k at 700.°c for the formation of ammonia?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be
Answers: 1

Chemistry, 22.06.2019 18:10
Areader can tell that the meaning of “obnoxious” will include “having the quality of something” because of the .a) prefix b)pronunciation c)suffix d) word root
Answers: 3

Chemistry, 23.06.2019 13:30
Use the periodic table to classify each of the elements below. cadmium (cd): vanadium (v): xenon (xe): iodine (i): potassium (k): strontium (sr):
Answers: 3

Chemistry, 23.06.2019 13:30
Asap a 50.0 ml soap bubble is blown in a 27.0°c room. it drifts out an open window and lands in a snow bank at -3.0°c. what is its new volume?
Answers: 1
You know the right answer?
Initially 2.0 moles of n2(g) and 4.0 moles of h2(g) were added to a 1.0-liter container and the foll...
Questions




English, 08.04.2020 07:19

Health, 08.04.2020 07:19

Mathematics, 08.04.2020 07:19


History, 08.04.2020 07:19

Biology, 08.04.2020 07:19

Biology, 08.04.2020 07:19


History, 08.04.2020 07:19

History, 08.04.2020 07:19


Social Studies, 08.04.2020 07:19

Mathematics, 08.04.2020 07:19

History, 08.04.2020 07:19

Computers and Technology, 08.04.2020 07:19


Mathematics, 08.04.2020 07:19

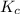 for the reaction is,
for the reaction is, 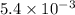
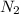 = 2.0 mol
= 2.0 mol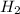 = 4.0 mol
= 4.0 mol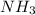 at equilibrium = 0.55 mol/L = 0.55 M
at equilibrium = 0.55 mol/L = 0.55 M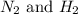 .
.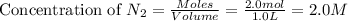

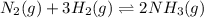
![K_c=\frac{[NH_3]^2}{[N_2][H_2]^3}](/tpl/images/0363/4597/c3aa0.png)
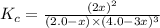 .......(1)
.......(1)



