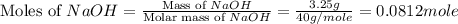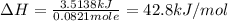Chemistry, 07.11.2019 05:31 heybrothwrlogan
When a 3.25 g sample of solid sodium hydroxide was dissolved in a calorimeter in 100.0 g of water, the temperature rose from 23.9 °c to 32.0 °c. calculate ∆h (in kj/mol) for the solution process: naoh (s) → na+ (aq) + oh- (aq)
use a calorimeter heat capacity of ccal = 15.8 j/°c

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:20
1. suppose a reaction mixture, when diluted with water, afforded 300 ml of an aqueous solution of 30 g of the reaction product malononitrile [ch2(cn)2], which is to be isolated by extraction with ether. the solubility of malononitrile in ether at room temperature is 20.0 g/100 ml, and in water is 13.3 g/100 ml. what weight of malononitrile would be recovered by extraction with (a) three 100-ml portions of ether and (b) one 300-ml portion of ether? suggestion: for each extraction, let x equal the weight extracted into the ether layer. in part (a), the concentration in the ether layer is x/100 and in the water layer is (30 x)/300; the ratio of these quantities is equal to k 20/13.3.
Answers: 2

Chemistry, 22.06.2019 07:00
At 450 mm hg a gas has a volume of 760 l, what is its volume at standard pressure
Answers: 2

Chemistry, 22.06.2019 08:00
This classification of drug typically changes the brain's chemistry and reduces its ability to create its own endorphins.
Answers: 1

Chemistry, 22.06.2019 15:00
Many ionic compounds and a few highly polar covalent compounds are because they completely ionize in water to create a solution filled with charged ions that can conduct an electric current.
Answers: 1
You know the right answer?
When a 3.25 g sample of solid sodium hydroxide was dissolved in a calorimeter in 100.0 g of water, t...
Questions

Mathematics, 04.03.2021 01:40

Mathematics, 04.03.2021 01:40

Mathematics, 04.03.2021 01:40



History, 04.03.2021 01:40

Mathematics, 04.03.2021 01:40

Medicine, 04.03.2021 01:40


Computers and Technology, 04.03.2021 01:40


History, 04.03.2021 01:40


English, 04.03.2021 01:40


History, 04.03.2021 01:40

Mathematics, 04.03.2021 01:40



![q=[q_1+q_2]](/tpl/images/0363/3965/341bc.png)
![q=[c_1\times \Delta T+m\times c_2\times \Delta T]](/tpl/images/0363/3965/21bf4.png)
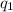 = heat absorbed by the calorimeter
= heat absorbed by the calorimeter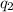 = heat absorbed by the water
= heat absorbed by the water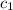 = specific heat of calorimeter =
= specific heat of calorimeter = 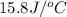
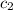 = specific heat of water =
= specific heat of water = 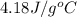
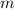 = mass of water = 100.0 g
= mass of water = 100.0 g = change in temperature =
= change in temperature = 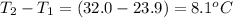
![q=[(15.8J/^oC\times 8.1^oC)+(100.0g\times 4.18J/g^oC\times 8.1^oC)]](/tpl/images/0363/3965/4042f.png)
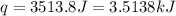 (1 kJ = 1000 J)
(1 kJ = 1000 J)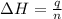
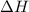 = enthalpy change = ?
= enthalpy change = ?