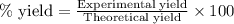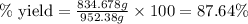Ether, (c2h5)2o, which was originally used as an anesthetic but has been replaced by safer and more effective medications, is prepared by the reaction of ethanol with sulfuric acid. what is the percent yield of ether if 1.17 l (d = 0.7134 g/ml) is isolated from the reaction of 1.500 l of c2h5oh (d = 0.7894 g/ml)? 2 c2h5oh + h2so4 → (c2h5)2o + h2so4 · h2o

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Complete the sentence. the lower the hydrogen ion concentration, the the ph. higher lower closer to 7 closer to 0
Answers: 2

Chemistry, 22.06.2019 09:00
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1

Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1

You know the right answer?
Ether, (c2h5)2o, which was originally used as an anesthetic but has been replaced by safer and more...
Questions

Mathematics, 12.05.2021 18:50



Mathematics, 12.05.2021 18:50




History, 12.05.2021 18:50

Chemistry, 12.05.2021 18:50

Mathematics, 12.05.2021 18:50

Mathematics, 12.05.2021 18:50


Mathematics, 12.05.2021 18:50

Mathematics, 12.05.2021 18:50





Mathematics, 12.05.2021 18:50

Mathematics, 12.05.2021 18:50

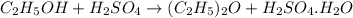
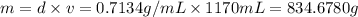
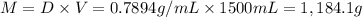
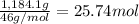
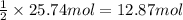 of an ether.
of an ether.