
Chemistry, 07.11.2019 02:31 whitwelllife
Pyridinium is a weak acid having a pka of 5.2. how much pyridine (the conjugate base of pyridinium) must be added to an aqueous solution of 0.100m pyridinium (assume the solution volume is constant) to make a buffer that is designed to keep the ph at 4.7?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:00
What happened in 2012 and how does it illustrate the importance of understanding the sun and how it works?
Answers: 3

Chemistry, 22.06.2019 11:30
Aperfume bottle is dropped in the corner of a room. the odor of the perfume can be detected on the other side of the room. which statement best describes this observation?
Answers: 2

Chemistry, 22.06.2019 15:30
Which suspect most likely committed the robbery and how do you know
Answers: 2

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3
You know the right answer?
Pyridinium is a weak acid having a pka of 5.2. how much pyridine (the conjugate base of pyridinium)...
Questions

English, 14.12.2019 20:31



Social Studies, 14.12.2019 20:31

Biology, 14.12.2019 20:31


Mathematics, 14.12.2019 20:31


Mathematics, 14.12.2019 20:31


History, 14.12.2019 20:31


Mathematics, 14.12.2019 20:31


Biology, 14.12.2019 20:31

History, 14.12.2019 20:31



Mathematics, 14.12.2019 20:31

![pH=pK_a+log\frac{[Conjugate\ base]}{[weak\ acid]}](/tpl/images/0363/0377/c0611.png)
![pH=pK_a+log\frac{[Py]}{PyH^+]}](/tpl/images/0363/0377/17718.png)
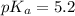
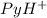 (Pyridinium)=0.100 M
(Pyridinium)=0.100 M![pH=pK_a+log\frac{[Py]}{PyH^+]}\\4.7=5.2 log\frac{[Py]}{0.100}](/tpl/images/0363/0377/de0ef.png)
![4.7-5.2=log\frac{[Py]}{0.100} \\-0.5=log\frac{[Py]}{0.100}\\\frac{[Py]}{0.100}=antilog -0.5\\\frac{[Py]}{0.100}=0.316](/tpl/images/0363/0377/5a83b.png)
![\frac{[Py]}{0.100} =0.316](/tpl/images/0363/0377/cf365.png)


