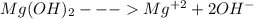Chemistry, 07.11.2019 01:31 shimmerandshine1
The ksp of magnesium hydroxide, mg(oh)2, is 1.2 x 10-11, so setting 4s3 = ksp, results in s = 1.4 x 10-4. this leads to an [oh-] = 2.8 x 10-4 (i. e., a poh=3.55), thus a ph of 10.45. given the aforementioned information and resulting calculations, comment on the solubility of mg(oh)2 at ph values: 1) less than 10.45 and, 2) greater than 10.45

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:10
What does a particular point on a line of a phase diagram represent? o a. the maximum temperature a substance can exist at without bonds breaking b. the pressure created by the kinetic energy of molecules at a particular temperature c. the melting point or boiling point of a substance at a specific pressure d. the conditions in which temperature and pressure have equal effects on a substance
Answers: 2

Chemistry, 21.06.2019 22:30
Determine the wavelength of the light absorbed when an electron in a hydrogen atom makes a transition from an orbital in the n=3 level to an orbital in the n=7 level.
Answers: 2

Chemistry, 22.06.2019 06:30
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1

Chemistry, 22.06.2019 07:00
Indicate whether the specified alkyl halides will form primarily substitution products, only elimination products, both substitution and elimination products, or no products when they react with sodium methoxide. 1-bromobutane 1-bromo-2-methylpropane 2-bromobutane 2-bromo-2-methylpropane
Answers: 2
You know the right answer?
The ksp of magnesium hydroxide, mg(oh)2, is 1.2 x 10-11, so setting 4s3 = ksp, results in s = 1.4 x...
Questions

Chemistry, 24.09.2021 23:30

Mathematics, 24.09.2021 23:30




Mathematics, 24.09.2021 23:30



History, 24.09.2021 23:30

History, 24.09.2021 23:30


History, 24.09.2021 23:30


Mathematics, 24.09.2021 23:30

Mathematics, 24.09.2021 23:30

Mathematics, 24.09.2021 23:30



Computers and Technology, 24.09.2021 23:30

Mathematics, 24.09.2021 23:30