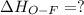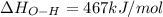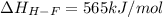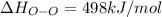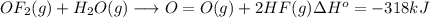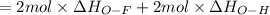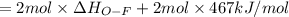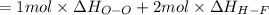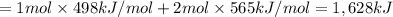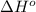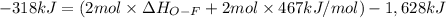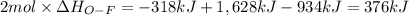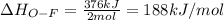Chemistry, 07.11.2019 01:31 jenlicavoli
Oxygen difluoride is an unstable molecule that reacts readily with water. calculate the bond energy of the o–f bond using the standard enthalpy of reaction and the bond energy data provided. just enter a number (no units). of2(g) + h2o(g) \longrightarrow⟶ o=o(g) + 2hf(g) \deltaδh° = –318 kj bond: o–h o=o h–f bond energy (kj/mol): 467 498 565

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:50
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

Chemistry, 22.06.2019 08:20
What is the formula for the compound dinitrogen pentoxide? a. n4o5 b. n5o4 c. n4o6 d. n5o2 e. n2o5
Answers: 3

Chemistry, 22.06.2019 10:00
The tendency of water molecules to stick together is referred to as a) adhesion b) polarity c) cohesion d) transpiration e) evaporation
Answers: 1

Chemistry, 22.06.2019 11:00
Which are examples of how technology has advanced scientific understanding.1using hot water to sterilize medical equipment.2transplanting a human organ into another individual.3inserting genes from one sheep into another cell to make a cloneunderstanding the different structures that make up a cell.4examining microorganisms from the deepest parts of the ocean
Answers: 2
You know the right answer?
Oxygen difluoride is an unstable molecule that reacts readily with water. calculate the bond energy...
Questions

English, 16.07.2019 23:00







Mathematics, 16.07.2019 23:00






Physics, 16.07.2019 23:00



History, 16.07.2019 23:00


Social Studies, 16.07.2019 23:00