
Chemistry, 06.11.2019 06:31 maggie123433
The aluminum cup inside your calorimeter weighs 41.55 g. you add 59.21 g of 1.0 m acetic acid solution and 50.03 g of 1.0 m sodium hydroxide solution to the calorimeter. both solutions have an initial temperature of 19.9 oc, and the final temperature after addition is 26.8 oc. what is the molar enthalpy of neutralization, in units of kj/mol? assume that: the calorimeter is completely insulated the heat capacity of the empty calorimeter is the heat capacity of the aluminum cup: 0.903 j g-1 oc-1. the density of the two solutions is the same as that of water: 1.00 g/ml. the heat capacity of the two solutions is the same as that of water: 4.184 j g-1 oc-1.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
The best solution for preventing harm to people and pets from severe hurricanes involves determining and warning residents about what
Answers: 1

Chemistry, 22.06.2019 07:10
Remember to use the proper number of significant figures and leading zeros in all calculations.gelatin has a density of 1.27 g/cm³. if you have a blob of gelatin dessert that fills a 2.0 liter bottle, what is its mass? 2540 g2500 g3.9 x 10-43.937x 10-4
Answers: 3

Chemistry, 22.06.2019 13:30
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1

Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3
You know the right answer?
The aluminum cup inside your calorimeter weighs 41.55 g. you add 59.21 g of 1.0 m acetic acid soluti...
Questions

Biology, 17.09.2019 23:00




Mathematics, 17.09.2019 23:00

Mathematics, 17.09.2019 23:00


German, 17.09.2019 23:00


Social Studies, 17.09.2019 23:00



Social Studies, 17.09.2019 23:00







Mathematics, 17.09.2019 23:00

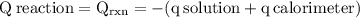
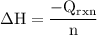
![\rm \DeltaH=-[\dfrac{- 3412.6007}{0.05003}]\\\\\Delta H=\:68211.087\dfrac{J}{mole} =68.211\dfrac{kJ}{mol}](/tpl/images/0361/7451/7b811.png)


