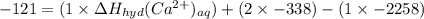Chemistry, 06.11.2019 02:31 wirchakethan23
The heat of solution of calcium chloride is -121 kj/mol. given that the lattice energy of calcium chloride is -2258 kj/mol and the heat of hydration of a chloride ion is -338 kj/mol calculate the heat of hydration of a calcium ion.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
What volume of a 2.00 m stock solution of naoh is needed to prepare 150. ml of 0.40 m solution?
Answers: 2

Chemistry, 22.06.2019 05:40
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3
Answers: 3

Chemistry, 22.06.2019 06:00
When a spring is compressed, the energy changes from kinetic to potential. which best describes what is causing this change?
Answers: 3

Chemistry, 22.06.2019 10:00
Water's surface tension and heat storage capacity are accounted for by its a) orbitals b) weight c) hydrogen bonds d) mass e) size
Answers: 2
You know the right answer?
The heat of solution of calcium chloride is -121 kj/mol. given that the lattice energy of calcium ch...
Questions

English, 16.03.2020 23:25





Computers and Technology, 16.03.2020 23:25





Mathematics, 16.03.2020 23:25


Mathematics, 16.03.2020 23:25





Social Studies, 16.03.2020 23:25


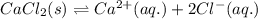
![\Delta H_{sol}=[1mol\times \Delta H_{hyd}(Ca^{2+})_{aq.}]+[2mol\times \Delta H_{hyd}(Cl^{-})_{aq.}]-[1mol\times U(CaCl_{2})_{s}]](/tpl/images/0361/2975/af20b.png)
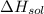 is heat of solution,
is heat of solution, 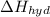 is heat of hydration and U represents lattice energy.
is heat of hydration and U represents lattice energy.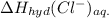 = -338 kJ/mol and
= -338 kJ/mol and 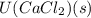 = -2258 kJ/mol
= -2258 kJ/mol