

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Which of the following pairs of elements belong to the same groupa. h and he b. li and bec. c and pb d. ga and ge
Answers: 1

Chemistry, 22.06.2019 10:00
Americium-241 undergoes fission to produce three neutrons per fission event. if a neutron-absorbing material is mixed in with this sample so that the rate of neutron production drops down to 1.8 neutrons per fission event, which will be effective at achieving a critical mass? check all that apply. remove a deflective shield surrounding the sample. remove absorbent material mixed in with the sample. compress the sample of americium-241.
Answers: 1

Chemistry, 22.06.2019 16:00
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1

Chemistry, 22.06.2019 16:30
4. a 20-kg child is tossed up into the air by her parent. the child is 2 meters off the ground traveling 5 m/s. circle one: ke / gpe / both show your work for finding the values of each type of energy the object has:
Answers: 1
You know the right answer?
10.0 g of gaseous ammonia and 6.50 g of oxygen gas are introduced into a previously evacuated 5.50 l...
Questions

Social Studies, 01.11.2019 14:31



Mathematics, 01.11.2019 14:31

Mathematics, 01.11.2019 14:31


Mathematics, 01.11.2019 14:31

History, 01.11.2019 14:31


Mathematics, 01.11.2019 14:31


History, 01.11.2019 14:31

Mathematics, 01.11.2019 14:31



Health, 01.11.2019 14:31

Biology, 01.11.2019 14:31


Mathematics, 01.11.2019 14:31

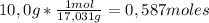
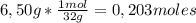
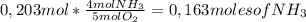
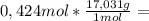 7,22g of NH₃
7,22g of NH₃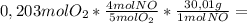 4,87g of NO
4,87g of NO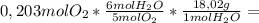 4,39g of H₂O
4,39g of H₂O

