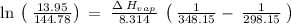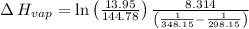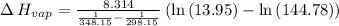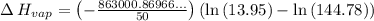Chemistry, 05.11.2019 06:31 rileyeddins1010
Gasoline is a mixture of hydrocarbons, a major component of which is octane, ch3ch2ch2ch2ch2ch2ch2ch3. octane has a vapor pressure of 13.95 torr at 25∘c and a vapor pressure of 144.78 torr at 75∘c. use these data and the equation in part (a) to calculate the heat of vaporization of octane.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:40
What are the resulting coefficients when you balance the chemical equation for the combustion of ethane, c2h6? in this reaction, ethane is burned in the presence of oxygen (o2) to form carbon dioxide (co2) and water (h2o). (g)+(g)→(g)+(g)
Answers: 1

Chemistry, 21.06.2019 18:00
Sylvanite is a mineral that contains 28.0% gold by mass. how much sylvanite would you need to dig up to obtain 77.0 g of gold? explain how you got your answer and the steps you took. you
Answers: 3

Chemistry, 21.06.2019 22:30
Ionic compounds are made of ions, and yet the overall charge of an ionic compound is neutral. why?
Answers: 1

You know the right answer?
Gasoline is a mixture of hydrocarbons, a major component of which is octane, ch3ch2ch2ch2ch2ch2ch2ch...
Questions


Mathematics, 12.10.2020 19:01

Mathematics, 12.10.2020 19:01

Mathematics, 12.10.2020 19:01




Mathematics, 12.10.2020 19:01

Mathematics, 12.10.2020 19:01

Mathematics, 12.10.2020 19:01

Mathematics, 12.10.2020 19:01

Mathematics, 12.10.2020 19:01

History, 12.10.2020 19:01


Mathematics, 12.10.2020 19:01




Mathematics, 12.10.2020 19:01

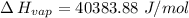
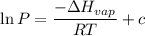
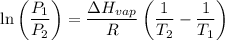
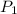 = 13.95 torr
= 13.95 torr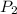 = 144.78 torr
= 144.78 torr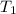 = 25°C
= 25°C
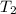 = 75°C = 348.15 K
= 75°C = 348.15 K