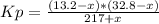An equilibrium mixture of pcl₅(g), pcl₃(g), and cl₂(g) has partial pressures of 217.0 torr, 13.2 torr, and 13.2 torr, respectively. a quantity of cl₂(g) is injected into the mixture, and the total pressure jumps to 263.0 torr. the appropriate chemical equation is pcl₃(g)+cl₂(g)↽−−⇀pcl₅(g) calculate the new partial pressures after equilibrium is reestablished.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
A100-watt light bulb radiates energy at a rate of 100 j/s. (the watt, a unit of power or energy over time, is defined as 1 j/s.) if all of the light emitted has a wavelength of 525 nm , how many photons are emitted per second?
Answers: 1

Chemistry, 22.06.2019 05:40
Consider the elements bromine and chlorine; which elements has a larger ionic radius ?
Answers: 1

Chemistry, 22.06.2019 07:30
In the particles are arranged in a regular, repeating pattern. a)a crystalline liquid b)a crystalline solid c)all gases d)all solids
Answers: 2

You know the right answer?
An equilibrium mixture of pcl₅(g), pcl₃(g), and cl₂(g) has partial pressures of 217.0 torr, 13.2 tor...
Questions



Mathematics, 23.07.2021 09:00


Computers and Technology, 23.07.2021 09:00



Computers and Technology, 23.07.2021 09:00

English, 23.07.2021 09:00


English, 23.07.2021 09:00

Health, 23.07.2021 09:00

Mathematics, 23.07.2021 09:00


Mathematics, 23.07.2021 09:00





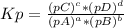 , where pX is the partial pressure of X.
, where pX is the partial pressure of X.