
Chemistry, 05.11.2019 03:31 paytonpaige22
Aluminum reacts with excess aqueous hydrochloric acid to produce hydrogen. (a) write a balanced chemical equation for the reaction. (hint: water-soluble alcl3 is the stable chloride of aluminum.) (b) calculate the mass of pure aluminum that will furnish 10.0 l hydrogen at a pressure of 0.750 atm and a temperature of 30.0°c.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Which formula can be used to calculate the molar mass of hydrogen peroxide
Answers: 1

Chemistry, 22.06.2019 10:50
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1

Chemistry, 23.06.2019 00:30
What are the advantages of using the metric system? designed as a decimal system making conversions simpler more accurate system of measurement has prefixes that correspond to an amount to use with all base units used by the entire scientific community
Answers: 2

You know the right answer?
Aluminum reacts with excess aqueous hydrochloric acid to produce hydrogen. (a) write a balanced chem...
Questions

Mathematics, 29.09.2019 23:10




English, 29.09.2019 23:10

Social Studies, 29.09.2019 23:10

Social Studies, 29.09.2019 23:10

Chemistry, 29.09.2019 23:10





Mathematics, 29.09.2019 23:10

Social Studies, 29.09.2019 23:10



History, 29.09.2019 23:10



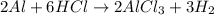
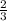 mole of aluminum is consumed.
mole of aluminum is consumed.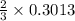 mole of aluminum is consumed.
mole of aluminum is consumed.


