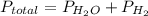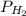Chemistry, 05.11.2019 02:31 jitosfc916
If the vapor pressure of water ( ) at 20.0°c is 17.5 mm hg, and the atmospheric pressure measured by a barometer (
) at 20.0°c is 17.5 mm hg, and the atmospheric pressure measured by a barometer ( ) was 757.3 mm hg, what is the partial pressure of h₂ gas (
) was 757.3 mm hg, what is the partial pressure of h₂ gas ( ) in the gas mixture in a buret? (use dalton’s law of partial pressures to solve for
) in the gas mixture in a buret? (use dalton’s law of partial pressures to solve for  )
)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:50
Working with si (metric) units for each of the following commonly used measurements, indicate its symbol. liter gram milliliter kilogram meter centigram milligram centimeter kilometer second millimeter milliseconds
Answers: 1

Chemistry, 22.06.2019 01:20
Match the acid base pairs by arranging the acid name with the conjugate base formula. hydrogen carbonate hydrogen phosphate carbonic acid read water sulfuric acid phosphoric acid a. co32- b. hso4- c. hco3- d. po43- e. h2po4- f. oh-
Answers: 1

Chemistry, 22.06.2019 17:00
The biosphere of the earth is made up of what compound? organic or inorganic?
Answers: 3

Chemistry, 22.06.2019 19:40
What causes different colors to appear in the sky? the absorption of light by air molecules the reflection of light by bodies of water the greenhouse effect in earth's atmosphere the scattering and reflection of light by dust particles
Answers: 2
You know the right answer?
If the vapor pressure of water ([tex]p_{h_2o}[/tex]) at 20.0°c is 17.5 mm hg, and the atmospheric pr...
Questions



Mathematics, 20.01.2021 18:00

Computers and Technology, 20.01.2021 18:00

Mathematics, 20.01.2021 18:00

Arts, 20.01.2021 18:00



History, 20.01.2021 18:00


Spanish, 20.01.2021 18:10



Mathematics, 20.01.2021 18:10


Mathematics, 20.01.2021 18:10




Mathematics, 20.01.2021 18:10