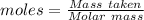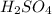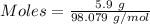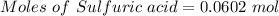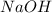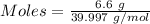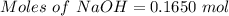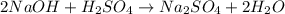Chemistry, 05.11.2019 00:31 safiyabrowne7286
Aqueous sulfuric acid (h2so4) reacts with solid sodium hydroxide (naoh) to produce aqeous sodium sulfate (na2so4) and liquid water (h2o). what is the theoretical yield of water formed from the reaction of 5.9 g of sulfuric acid and 6.6 g of sodium hydroxide?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Liv sheldon given the balanced equation for an organic reaction: c2h2 + 2cl2 → c2h2cl4 this reaction is best classified as *
Answers: 1

Chemistry, 22.06.2019 11:40
Calculate the number of kilojoules to warm 125 g of iron from 23.5°c to 78.0°c.
Answers: 3


Chemistry, 22.06.2019 17:30
Air can be considered a mixture. which statement does not explain why?
Answers: 1
You know the right answer?
Aqueous sulfuric acid (h2so4) reacts with solid sodium hydroxide (naoh) to produce aqeous sodium sul...
Questions



Biology, 17.03.2021 23:40

Mathematics, 17.03.2021 23:40

Mathematics, 17.03.2021 23:40

History, 17.03.2021 23:40

History, 17.03.2021 23:40


Mathematics, 17.03.2021 23:40

English, 17.03.2021 23:40

Mathematics, 17.03.2021 23:40

Chemistry, 17.03.2021 23:40


Mathematics, 17.03.2021 23:40

Mathematics, 17.03.2021 23:40

History, 17.03.2021 23:40