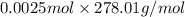Chemistry, 04.11.2019 23:31 pinkycupcakes3oxbqhx
Apowder contains feso4⋅7h2o (molar mass=278.01 g/mol), among other components. a 2.955 g sample of the powder was dissolved in hno3 and heated to convert all iron to fe3+. the addition of nh3 precipitated fe2o3⋅xh2o, which was subsequently ignited to produce 0.201 g fe2o3. what was the mass of feso4⋅7h2o in the 2.955 g sample?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 21:30
What is another way to determine mass times acceleration?
Answers: 1

Chemistry, 23.06.2019 04:00
The movement of tectonic plates and in two locations is described below: location a: tectonic played push together location b: tectonic plates push apart
Answers: 1

Chemistry, 23.06.2019 08:30
Benzonitrile (c6h5cn) is reduced to two different products depending on the reducing agent used. treatment with lithium aluminum hydride followed by water forms k, which has a molecular ion in its mass spectrum at 107 and the following ir absorptions: 3373, 3290, 3062, 2920, and 1600 cm-1. treatment with a milder reducing agent forms l, which has a molecular ion in its mass spectrum at 106 and the following ir absorptions: 3086, 2850, 2820, 2736, 1703, and 1600 cm-1. l shows fragments in its mass spectrum at m/z = 105 and 77. propose structures for k and l and choose an explanation for how this could be concluded.
Answers: 3

Chemistry, 23.06.2019 11:20
When using the ideal gas law constant 0.0821, what unit is used for volume? a) galloonb) ouncec) milliliterd) liter
Answers: 1
You know the right answer?
Apowder contains feso4⋅7h2o (molar mass=278.01 g/mol), among other components. a 2.955 g sample of t...
Questions

Mathematics, 15.04.2020 20:26









Physics, 15.04.2020 20:26










Geography, 15.04.2020 20:27

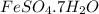 =
= 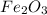
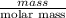
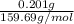
 mol
mol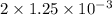 mol
mol