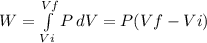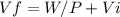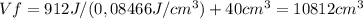Consider a mixture of air and gasoline vapor in a cylinder with a piston. the original volume is 40. cm3. if the combustion of this mixture releases 912 j of energy, to what volume will the gases expand against a constant pressure of 635 torr if all the energy of combustion is converted into work to push back the piston?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 14:30
An object resting on a table weighs 100 n. with what force is the object pushing on the table? with what force is the table pushing on the object? explain how you got your answer.
Answers: 3

Chemistry, 22.06.2019 20:00
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2

Chemistry, 22.06.2019 21:50
Liquid from a brewery fermentation contains 10% ethanol and 90% water. part of the fermentation product (50,000 kg/h) is pumped to a distillation column on the factory site. under current operating conditions, a distillate of 45% ethanol and 55% water is produced from the top of the column at a rate of one-tenth that of the feed. what is the composition of the waste "bottoms" from the still?
Answers: 2
You know the right answer?
Consider a mixture of air and gasoline vapor in a cylinder with a piston. the original volume is 40....
Questions



Mathematics, 09.07.2019 03:00

Spanish, 09.07.2019 03:00





Chemistry, 09.07.2019 03:00


Chemistry, 09.07.2019 03:00

Mathematics, 09.07.2019 03:00

Physics, 09.07.2019 03:00



History, 09.07.2019 03:00


Chemistry, 09.07.2019 03:00


Mathematics, 09.07.2019 03:00