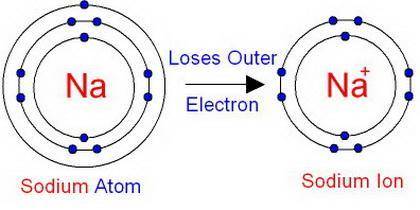
How does an ion differ from an electrically-neutral atom?
a) there are a different number of...

Chemistry, 03.11.2019 07:31 nayelidlc2
How does an ion differ from an electrically-neutral atom?
a) there are a different number of protons in an ion compared to a neutral atom. b) there are a different number of electrons in an ion compared to a neutral atom. c) there a different number of neutrons in an ion compared to a neutral atom.
d) there are no differences in subatomic particles.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What must happen before a body cell can begin mitotic cell division
Answers: 2

Chemistry, 22.06.2019 03:30
Explain why pure hydrogen cyanide does not conduct electricity, but become a conductor when it is dissolved in water? (at room temp, pure hcn exists as a volatile liquid)
Answers: 1


Chemistry, 23.06.2019 01:30
Which of the following statements is true about energy quantization at the atomic level? electrons in the outermost orbits are the most stable. electrons in all the orbits around the nucleus have the same amount of energy. electrons in the orbit closest to the nucleus have the least amount of energy. electrons absorb or release the same amount of energy independent of the energy levels.
Answers: 1
You know the right answer?
Questions

English, 12.04.2021 20:10


Mathematics, 12.04.2021 20:10

Business, 12.04.2021 20:10




Mathematics, 12.04.2021 20:10


Mathematics, 12.04.2021 20:10

History, 12.04.2021 20:10





Chemistry, 12.04.2021 20:10

Social Studies, 12.04.2021 20:10



Mathematics, 12.04.2021 20:10