
Chemistry, 02.11.2019 07:31 morganhines181
Solution of the schrödinger wave equation for the hydrogen atom results in a set of functions (orbitals) that describe the behavior of the electron. each function is characterized by 3 quantum numbers: n, l, and ml. if the value of n = 1 the quantum number l can have values from to . the total number of orbitals possible at the n = 1 energy level is . if the value of l = 1 the quantum number ml can have values from to . the total number of orbitals possible at the l = 1 sublevel is .

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:00
What mass of carbon dioxide is produced from the complete combustion of 4.50×10−3 g of methane?
Answers: 2


Chemistry, 22.06.2019 00:30
What is the most stable monatomic ion formed from nitrogen
Answers: 2

Chemistry, 22.06.2019 06:30
Select the correct text in the passage. which sentences describe examples of sustainable living? i live in an old apartment building downtown, but my company is based in an office park on the outskirts of the city. i drive an old car that needs to be replaced. i plan to buy a hybrid for better gas mileage, but for now i am able to carpool with a couple of friends from work. the drive to the office park is about 45 minutes each way, but we do get to work in a modern building. the architects just received a leed certification for the design.
Answers: 3
You know the right answer?
Solution of the schrödinger wave equation for the hydrogen atom results in a set of functions (orbit...
Questions


Mathematics, 05.03.2020 22:20




Mathematics, 05.03.2020 22:21



Mathematics, 05.03.2020 22:21






Mathematics, 05.03.2020 22:22

Computers and Technology, 05.03.2020 22:22




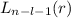 , being n and l the quantum numbers. As is known, in the Laguerre polynomials the subindex must be greater than or equal to 0, which implies
, being n and l the quantum numbers. As is known, in the Laguerre polynomials the subindex must be greater than or equal to 0, which implies 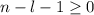 . So if n=1, the only possible value of l is l=0.
. So if n=1, the only possible value of l is l=0. ![Y_{l,m_l}(\theta,\phi)[\tex] as solution. One of the properties of spherical harmonics is that the number [tex]m_l[\tex] can only take the values [tex]m_l=-l,-l+1,-l+2,...,l-1,l[\tex]. So if l=0, as above, [tex]m_l[\tex] can only take the value [tex]m_l=0,[\tex] thus giving only 1 orbital. But if l=1, [tex]m_l](/tpl/images/0356/6953/a304c.png) can be
can be 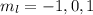 . Therefore the sublevel l=| has 3 possible orbitals.
. Therefore the sublevel l=| has 3 possible orbitals.

