

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
In the millikan oil drop experiment they determined that every drop had a charge which was a while number multiple of -1.60x10^-19. if a drop has a total charge of -9.60x10^-19 then how many excess electrons are contained within the drop?
Answers: 2

Chemistry, 22.06.2019 13:30
1) which of the following is the best example of a physical change? a) sugar dissolving in tea b) firefly glowing 2) in the combustion of ethane, what is/are the reactants? c2h6 + o2 ==> co2 + h2o a) c2h6 and o2 b) co2 and c2h6
Answers: 2

Chemistry, 22.06.2019 20:00
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1

Chemistry, 22.06.2019 21:30
How can the periodic table be used to predict the behavior of elements?
Answers: 1
You know the right answer?
Hydrogen sulfide gas gives rotten eggs their terrible odor. hydrogen sulfide burns in air (o2) to pr...
Questions

French, 26.04.2021 22:30








History, 26.04.2021 22:30

Mathematics, 26.04.2021 22:30






English, 26.04.2021 22:30




Arts, 26.04.2021 22:30

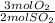 = 2.07 mol O₂
= 2.07 mol O₂

