
Chemistry, 02.11.2019 05:31 itsme123427
Aprotein was previously determined to contain 15.7 wt% nitrogen. a 647 m aliquot of a solution containing the protein was digested in boiling sulfuric acid. the solution was made basic and the liberated nh3 was collected in 10.00 ml of 0.0388 m hci. 3.83 ml of 0.0196 m naoh was required to react with the excess, unreacted hcl. calculate the protein concentration of the solution in units of milligrams per milliliter. number 0 mg protein ml solution

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:40
How many grams of aluminum will there be in 98g of al2o3?
Answers: 1

Chemistry, 22.06.2019 14:30
Chemistry worksheet - i am not sure what they are asking for exactly?
Answers: 1

Chemistry, 22.06.2019 19:20
Anyone who's in connections academy chemistry b have the factors that affect the rate of a reaction portfolio already done?
Answers: 3

Chemistry, 22.06.2019 19:30
Which one of the following substances would be the most soluble in ccl4? na2so4 h2o ch3ch2ch2ch2oh c4h10 hi
Answers: 1
You know the right answer?
Aprotein was previously determined to contain 15.7 wt% nitrogen. a 647 m aliquot of a solution conta...
Questions







Mathematics, 12.05.2021 04:00

Mathematics, 12.05.2021 04:00



Social Studies, 12.05.2021 04:00




Biology, 12.05.2021 04:00


Mathematics, 12.05.2021 04:00

Mathematics, 12.05.2021 04:00


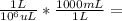 0.647 mL27.91 mg / 0.647 mL = 431.38 mg/mL
0.647 mL27.91 mg / 0.647 mL = 431.38 mg/mL


