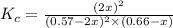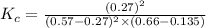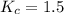Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
1. calculate the approximate enthalpy of the reaction in joules. estimate that 1.0 ml of vinegar has the same thermal mass as 1.0 ml of water. iqnore the thermal mass of th sodium bicarbonate. note: it takes about 4.2 joules () to change 1.0 gram (1.0ml) of water 1.0 c
Answers: 2


Chemistry, 22.06.2019 04:30
Suppose that during that icy hot lab 65,000 j of energy were transferred to 450 g of water at 20°c what would have have been the final temperature of the water
Answers: 2

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 3
You know the right answer?
Consider the reaction between no and cl2 to form nocl: 2no(g)+cl2(g)⇌2nocl(g) a reaction mixture at...
Questions

Biology, 03.12.2020 02:10

English, 03.12.2020 02:10

Mathematics, 03.12.2020 02:10


Mathematics, 03.12.2020 02:10

Health, 03.12.2020 02:10

Mathematics, 03.12.2020 02:10


Computers and Technology, 03.12.2020 02:10

History, 03.12.2020 02:10

Mathematics, 03.12.2020 02:10

Mathematics, 03.12.2020 02:10


History, 03.12.2020 02:10


Computers and Technology, 03.12.2020 02:10


Mathematics, 03.12.2020 02:10

Mathematics, 03.12.2020 02:10

History, 03.12.2020 02:10

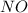 = 0.57 M
= 0.57 M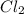 = 0.66 M
= 0.66 M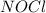 = 0.27 M
= 0.27 M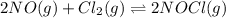
![K_c=\frac{[NOCl]^2}{[NO]^2[Cl_2]}](/tpl/images/0356/4959/56950.png)