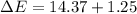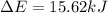Chemistry, 02.11.2019 03:31 Jsanders2276
In a piston, the addition of 14.37 kj of heat to a 100. g sample of a liquid at a constant temperature of 35.2 °c caused the liquid to vaporize (change to a gas). the vaporized gas expanded against an external pressure of 1.07 atm and a volume change of 11.49 l was observed. (recall: 1 l• atm = 101.3 j} what was the change in the internal energy of the system, (ae in kj)? (enter your answer with two decimals places and no units.)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:00
During which movies do spring tides new moon first quarter waxing gibbous waxing
Answers: 1

Chemistry, 21.06.2019 19:00
Identify which properties could correspond to solids, plasmas, or both. maintain a unique shape. collide infrequently with other particles. have very high velocities. conduct electricity. protons. have a low temperature. has long-range order.
Answers: 1

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2

Chemistry, 22.06.2019 09:30
Which ocean zone has the most abundant primary producer and why a) the abyssopelagic zone ,du to the absence of light and cold water temperatureb) the bathypelagic zone, due to the absence of light and cold water temperaturec) the mesopelagic zone ,due to uts high light availability and warm water temperature d) the epipelagic zone,due to its high light availability and warm water temperature
Answers: 3
You know the right answer?
In a piston, the addition of 14.37 kj of heat to a 100. g sample of a liquid at a constant temperatu...
Questions


Health, 18.11.2020 07:30



SAT, 18.11.2020 07:30




Business, 18.11.2020 07:30






Mathematics, 18.11.2020 07:30


History, 18.11.2020 07:30

Biology, 18.11.2020 07:30

Biology, 18.11.2020 07:30

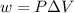
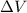 = change in volume = 11.49 L
= change in volume = 11.49 L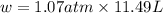
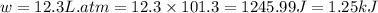 (as per conversion)
(as per conversion)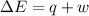
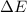 = internal energy of the system
= internal energy of the system