
Oxygen gas can be prepared by heating potassium chlorate according to the following equation: 2kclo3(s)2kcl(s) + 3o2(g) the product gas, o2, is collected over water at a temperature of 25 °c and a pressure of 749 mm hg. if the wet o2 gas formed occupies a volume of 5.76 l, the number of moles of kclo3 reacted was mol. the vapor pressure of water is 23.8 mm hg at 25 °c.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:00
Drag each tile to the correct box arrange the layers not order from oldest to youngest
Answers: 2

Chemistry, 22.06.2019 08:30
Agroup of students is studying convection current. they fill two identical balloons with the same amount of helium. one balloon is placed in a freezer and the other is in an area with warm air. after 10 minutes, the balloon are released from a height of 1 meter. which of the following to the students most likely observe? a) the warm balloon expands and rises. the cold balloon shrinks and sinks b) the balloon both rise. the cold balloon is larger than the warm balloon c) the cold balloon expands and rises. the warm balloon shrinks and sinks d) the balloon rise at the same rate. both balloons are the same size
Answers: 1

Chemistry, 22.06.2019 23:00
What extra step distinguishes fermentation from glycolysis
Answers: 1

Chemistry, 22.06.2019 23:30
Imagine a small synthetic vesicle made from pure phospholipids enclosing an interior lumen containing 1 mm glucose and 1 mm sodium chloride. if the vesicle is placed in pure water, which of the following happens faster? a. na+ diffuses out. b. cl– diffuses out. c. h2o diffuses in. d. glucose diffuses out. e. sodium chloride diffuses out.
Answers: 3
You know the right answer?
Oxygen gas can be prepared by heating potassium chlorate according to the following equation: 2kclo...
Questions


Physics, 30.09.2019 10:10

Social Studies, 30.09.2019 10:10


Geography, 30.09.2019 10:10





Business, 30.09.2019 10:10

Biology, 30.09.2019 10:10



Mathematics, 30.09.2019 10:10

Physics, 30.09.2019 10:10

History, 30.09.2019 10:10

Social Studies, 30.09.2019 10:10

Mathematics, 30.09.2019 10:10

Mathematics, 30.09.2019 10:10

Social Studies, 30.09.2019 10:10

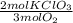 = 0,15 mol of KClO₃
= 0,15 mol of KClO₃

