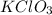Given the balanced equation representing a reaction:
2kclo3(s) ==> 2kcl(s) + 3o2(g)
...

Chemistry, 25.08.2019 04:00 jslaughter3
Given the balanced equation representing a reaction:
2kclo3(s) ==> 2kcl(s) + 3o2(g)
the oxidation state of chlorine in this reaction changes from
(1) -1 to +1 (3) +1 to -1
(2) -1 to +5 (4) +5 to -1

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:40
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2

Chemistry, 23.06.2019 02:00
When an experimenter draws a conclusion that he assumes will apply to all situations set up similarly to his test situation, even though he cannot possibly have examined all possible test scenarios, the experimenter is using deductive reasoning inductive reasoning abductive reasoning subjective reasoning
Answers: 1

Chemistry, 23.06.2019 10:50
Gene expression control that occurs during the generation of rna is a. controlled at transcription b. control before transcription c. controlled after transcription d. controlled after translation
Answers: 3

Chemistry, 23.06.2019 15:30
Sodium chloride can be made as follows: 2na + cl2 ? 2nacl i calculate the maximum amount of nacl possible if 2.3 g of sodium was reacted with excess chlorine. show all your workings.
Answers: 3
You know the right answer?
Questions

Chemistry, 25.08.2019 05:30

Mathematics, 25.08.2019 05:30




History, 25.08.2019 05:30

Mathematics, 25.08.2019 05:30

Biology, 25.08.2019 05:30

English, 25.08.2019 05:30



History, 25.08.2019 05:30

Biology, 25.08.2019 05:30



Chemistry, 25.08.2019 05:30


Physics, 25.08.2019 05:30

Social Studies, 25.08.2019 05:30