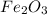Given the balanced equation representing a reaction:
fe2o3 + 2al--> al2o3 + 2fe
dur...

Chemistry, 24.01.2020 14:31 goverton101
Given the balanced equation representing a reaction:
fe2o3 + 2al--> al2o3 + 2fe
during this reaction, the oxidation number of fe changes from
(1) +2 to 0 as electrons are transferred
(2) +2 to 0 as protons are transferred
(3) +3 to 0 as electrons are transferred
(4) +3 to 0 as protons are transferred

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 05:30
Which other elements contain the same number of outer electrons as sodium
Answers: 3

Chemistry, 22.06.2019 14:30
According to le chatelier’s principle, a system in chemical equilibrium responds to stress by shifting the equilibrium in a direction that reduces the stress. normalizes the stress. increases the stress. changes the stress.
Answers: 1

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 1
You know the right answer?
Questions


Mathematics, 05.10.2019 19:00

Arts, 05.10.2019 19:00

History, 05.10.2019 19:00


English, 05.10.2019 19:00

Spanish, 05.10.2019 19:00


Chemistry, 05.10.2019 19:00

History, 05.10.2019 19:00


Spanish, 05.10.2019 19:00



Biology, 05.10.2019 19:00

Mathematics, 05.10.2019 19:00


Chemistry, 05.10.2019 19:00