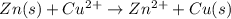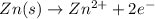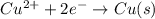Given the balanced ionic equation:
zn(s) + cu2+(aq) → zn2+(aq) + cu(s)
which equation r...

Chemistry, 22.01.2020 03:31 alyssasnyderrr
Given the balanced ionic equation:
zn(s) + cu2+(aq) → zn2+(aq) + cu(s)
which equation represents the oxidation half reaction?
(1) zn(s) + 2e– → zn2+(aq)
(2) zn(s) → zn2+(aq) + 2e–
(3) cu2+(aq) → cu(s) + 2e–
(4) cu2+(aq) + 2e– → cu(s)

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 13:00
12. calculate the hydroxide ion concentration of a solution with ph = 3.25. show all calculations leading to an answer
Answers: 3

Chemistry, 22.06.2019 13:50
Amap that uses a range of colors and shading to represent the elevation, depth, or landscape of specific features on earth is a/an map.
Answers: 3

Chemistry, 22.06.2019 16:00
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3
You know the right answer?
Questions


Mathematics, 29.10.2020 17:40

Chemistry, 29.10.2020 17:40


Mathematics, 29.10.2020 17:40

Mathematics, 29.10.2020 17:40

History, 29.10.2020 17:40


Arts, 29.10.2020 17:40



Chemistry, 29.10.2020 17:40


Mathematics, 29.10.2020 17:40

English, 29.10.2020 17:40

Chemistry, 29.10.2020 17:40

Mathematics, 29.10.2020 17:40

Mathematics, 29.10.2020 17:40


Chemistry, 29.10.2020 17:40