If the aluminum block is initially at 25 ∘c, what is the final temperature of the block after the evaporation of the alcohol? assume that the heat required for the vaporization of the alcohol comes only from the aluminum block and that the alcohol vaporizes at 25 ∘c. the heat of vaporization of the alcohol at 25 ∘c is 45.4 kj/mol, the specific heat of aluminum is 0.903 j/g⋅∘c

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 16:00
How could a student test the effect of removing heat from a gas that is stored in a sealed container? what must occur in order for matter to change states?
Answers: 2

Chemistry, 22.06.2019 16:30
An atom with 7 protons, 6 neutrons, and 7 electrons has an atomic mass of amu. (enter a whole number.) numerical answers expected! answer for blank 1:
Answers: 3

Chemistry, 22.06.2019 21:30
What is happening when the water inside a kettle heats up and begins to boil
Answers: 1

Chemistry, 23.06.2019 00:30
An ice cube with a volume of 45.0ml and a density of 0.9000g/cm3 floats in a liquid with a density of 1.36g/ml. what volume of the cube is submerged in the liquid
Answers: 3
You know the right answer?
If the aluminum block is initially at 25 ∘c, what is the final temperature of the block after the ev...
Questions

History, 26.08.2019 22:30

Chemistry, 26.08.2019 22:30


Biology, 26.08.2019 22:30





History, 26.08.2019 22:30

Biology, 26.08.2019 22:30

German, 26.08.2019 22:30

Physics, 26.08.2019 22:30



English, 26.08.2019 22:30

Health, 26.08.2019 22:30

Mathematics, 26.08.2019 22:30


Biology, 26.08.2019 22:30

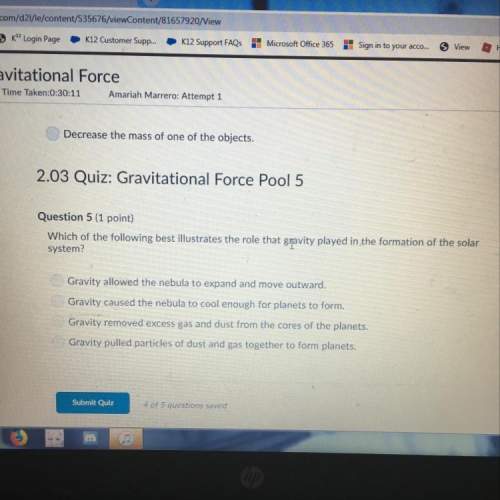
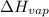 ) = 45.4 kJ/mol
) = 45.4 kJ/mol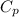 ) = 0.903
) = 0.903 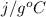
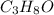 and its mass is 1.12 g. Also, mass of aluminium block is 73.0 g.
and its mass is 1.12 g. Also, mass of aluminium block is 73.0 g.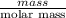
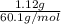
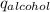 ) = heat lost by aluminium (
) = heat lost by aluminium (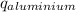 )
)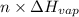 =
= 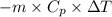
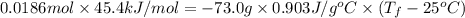
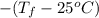
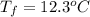
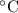 .
.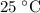
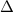 H)= 45.04 kJ/mol
H)= 45.04 kJ/mol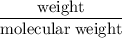
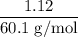
 45.04 kJ/mol
45.04 kJ/mol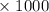 J = - 73
J = - 73