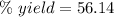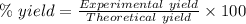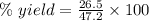Chemistry, 01.11.2019 03:31 tamikagoss22
The theoretical yield of a reaction can be determined using its in the reaction between co and fe3o4, the theoretical yield in an experiment is calculated to be 47.2 g fe. when a careless chemistry student carries out the experiment, the actual yield is 26.5 g fe. calculate the percentage yield. chemical equation and the starting amounts of the reactants.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:00
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 12:40
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1

Chemistry, 22.06.2019 19:00
Nan element’s square on the periodic table, the number with the greatest numerical value represents the
Answers: 3

Chemistry, 23.06.2019 00:30
On the periodic table, elements are arranged by which of the following. a. mass numbers. b. increasing atomic number. c. alphabetical order. or d. density
Answers: 1
You know the right answer?
The theoretical yield of a reaction can be determined using its in the reaction between co and fe3o4...
Questions

Medicine, 04.06.2021 20:40

Social Studies, 04.06.2021 20:40

Biology, 04.06.2021 20:40

Computers and Technology, 04.06.2021 20:40


Mathematics, 04.06.2021 20:40





Mathematics, 04.06.2021 20:40

Health, 04.06.2021 20:40



English, 04.06.2021 20:40

English, 04.06.2021 20:40


Mathematics, 04.06.2021 20:40

Mathematics, 04.06.2021 20:40

Mathematics, 04.06.2021 20:40