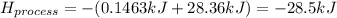Chemistry, 01.11.2019 02:31 tintlemax6256
When 50.0 ml of water containing 0.50 mol hcl at 22.5°c are mixed with 50.0 ml of water containing 0.50 mol naoh at 22.5°c in a calorimeter, the temperature of the solution increases to 26.0°c. how much heat (in kj) was released by this reaction? note: the specific heat of water (cwater) is 4.18 j/(g•˚c) and the density of the solution is 1.00 g/ml.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:00
Imagine that twenty i.u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2

Chemistry, 22.06.2019 14:30
Chemistry worksheet - i am not sure what they are asking for exactly?
Answers: 1


Chemistry, 22.06.2019 22:30
Amedication is given at a dosage of 3.000 mg of medication per kg of body weight. if 0.1500 g of medication is given, then what was the patient's weight in pounds (lbs)? there are 453.59g in 1 lb.
Answers: 2
You know the right answer?
When 50.0 ml of water containing 0.50 mol hcl at 22.5°c are mixed with 50.0 ml of water containing 0...
Questions









Mathematics, 04.07.2021 01:10


Mathematics, 04.07.2021 01:10



Social Studies, 04.07.2021 01:10

Physics, 04.07.2021 01:10

Mathematics, 04.07.2021 01:10

Mathematics, 04.07.2021 01:10

Physics, 04.07.2021 01:10


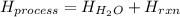
![H_{H_2O}=[(50.0mL+50mL)*\frac{1g}{1mL}]*4.18\frac{J}{mol^0C}*(26.0-22.5)^0C\\H_{H_2O}=146.3J=0.1463kJ](/tpl/images/0355/0596/d18d3.png)