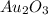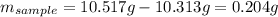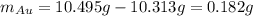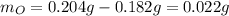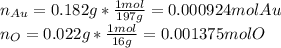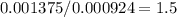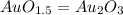Acompound is known to contain only gold and oxygen. a sample of this compound is placed in a clean crucible that has a mass of 10.313 g. the crucible and sample have a mass of 10.517 g. the crucible is heated until the compound decomposes to the elements. the oxygen is lost to the air and the gold remains in the crucible. the mass of the crucible and gold is 10.495 g. what is the empirical formula of this compound?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
How many moles are in 250 grams of tungsten (w)? * 4.4x10^23 moles 4.2x10^23 moles 0.7 moles 1.4 moles
Answers: 3

Chemistry, 22.06.2019 04:50
Write the overall equation for the reaction for lithium battery
Answers: 2

Chemistry, 22.06.2019 09:30
Apump contains 0.5 l of air at 203 kpa.you draw back on the piston of the pump, expanding the volume until the pressure reads 50.8 kpa. what is the new volume of the air pump
Answers: 2

Chemistry, 22.06.2019 17:00
The biosphere of the earth is made up of what compound? organic or inorganic?
Answers: 3
You know the right answer?
Acompound is known to contain only gold and oxygen. a sample of this compound is placed in a clean c...
Questions


World Languages, 05.05.2020 10:00


History, 05.05.2020 10:00




Biology, 05.05.2020 10:00

Mathematics, 05.05.2020 10:00




English, 05.05.2020 10:00

World Languages, 05.05.2020 10:00

Social Studies, 05.05.2020 10:00


Biology, 05.05.2020 10:00