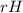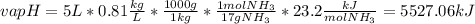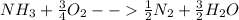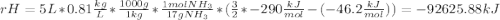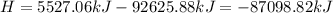Ammonia (nh3) boils at -33∘c; at this temperature it has a density of 0.81 g/cm3. the enthalpy of formation of nh3(g) is -46.2 kj/mol, and the enthalpy of vaporization of nh3(l) is 23.2 kj/mol calculate the enthalpy change when 5 l of liquid nh3 is burned in air to give n2(g) and h2o(g).

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 22.06.2019 22:10
What is the indicator of the number of ions in solution? the amount of conductivity the amount of precipitate the amount of solute added
Answers: 1

Chemistry, 23.06.2019 00:30
Ok, so i have 2 questions. try to answer them both: (the topic is fire) 1) how can you represent the chemical reaction of fire? 2) what kind of bond is formed in this chemical reaction
Answers: 3

Chemistry, 23.06.2019 01:00
Which description best characterization the motion of particles in a solid
Answers: 1
You know the right answer?
Ammonia (nh3) boils at -33∘c; at this temperature it has a density of 0.81 g/cm3. the enthalpy of f...
Questions



Social Studies, 16.09.2019 15:30




Computers and Technology, 16.09.2019 15:30

Mathematics, 16.09.2019 15:30

Arts, 16.09.2019 15:30

Mathematics, 16.09.2019 15:30

Geography, 16.09.2019 15:30

Chemistry, 16.09.2019 15:30

Chemistry, 16.09.2019 15:30


English, 16.09.2019 15:30


Advanced Placement (AP), 16.09.2019 15:30

Health, 16.09.2019 15:30


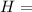 Δ
Δ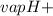 Δ
Δ