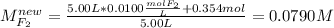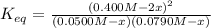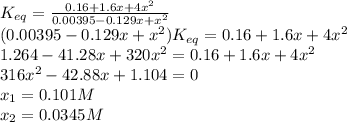Chemistry, 01.11.2019 02:31 krandall232
For the reaction below at a certain temperature, it is found that the equilibrium concentrations in a 5.00 l rigid container are [h2] = 0.0500 m, [f2] = 0.0100 m, and [hf] = 0.400 m. if 0.345 mol of f2 is added to this equilibrium mixture, calculate the concentrations of all gases once equilibrium is reestablished in moles/liter. h2(g) + f2(g) < > 2 hf(g)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which solution is an example of a nonelectrolyte? a. iodine in hexane b. sodium nitrate in waterc. acetic acid in waterd. hydrogen chloride in water
Answers: 2


Chemistry, 22.06.2019 09:40
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2

Chemistry, 23.06.2019 03:30
If you need to add 27.50ml of a solution, which piece of glassware would you use to deliver this volume and explain how you would determine if the 27.50 ml was measured?
Answers: 1
You know the right answer?
For the reaction below at a certain temperature, it is found that the equilibrium concentrations in...
Questions



Physics, 11.03.2021 05:50


Business, 11.03.2021 05:50



Mathematics, 11.03.2021 05:50

Mathematics, 11.03.2021 05:50

English, 11.03.2021 05:50

Mathematics, 11.03.2021 05:50

English, 11.03.2021 05:50

Mathematics, 11.03.2021 06:00




History, 11.03.2021 06:00


Social Studies, 11.03.2021 06:00

Mathematics, 11.03.2021 06:00

![[HF]_{eq}=0.469M, [H_2]_{eq}=0.0155M, [F_2]_{eq}=0.0445M](/tpl/images/0355/0697/87639.png)
![K_{eq}=\frac{[HF]^2}{[H_2][F_2]}=\frac{(0.400M)^2}{(0.0500M)(0.0100M)} =320](/tpl/images/0355/0697/ba1f2.png)