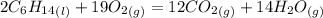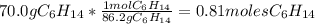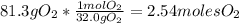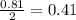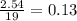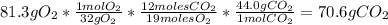Chemistry, 01.11.2019 02:31 twistedgamerhd12
Problem page liquid hexane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water . suppose 70. g of hexane is mixed with 81.3 g of oxygen. calculate the maximum mass of carbon dioxide that could be produced by the chemical reaction. be sure your answer has the correct number of significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Sex cells from female reproductive organ? 1) mitosis 2) fertilization 3) zygote 4) eggs 5) meiosis 6) sperm
Answers: 2

Chemistry, 22.06.2019 02:00
In which of these cases are the two wave points considered to be in phase with each other?
Answers: 1

Chemistry, 22.06.2019 03:20
What is the ima of the 1 st class lever in the graphic given? 2 3 0.5
Answers: 1

Chemistry, 22.06.2019 10:20
Gwhich r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? which r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? −ch2−oh −ch2−o||c−nh2 −ch2−coo− −ch2−ch2−ch2−ch2−n+h3
Answers: 3
You know the right answer?
Problem page liquid hexane will react with gaseous oxygen to produce gaseous carbon dioxide and gase...
Questions

Mathematics, 24.11.2021 21:00

History, 24.11.2021 21:00

Social Studies, 24.11.2021 21:00


Mathematics, 24.11.2021 21:00


Mathematics, 24.11.2021 21:00



Social Studies, 24.11.2021 21:00






Geography, 24.11.2021 21:00

English, 24.11.2021 21:00