
Chemistry, 01.11.2019 02:31 santileiva123199
Barium can be analyzed by precipitating it as baso4 and determining the mass of the precipitate. when a 0.269 g sample of a barium compound was treated with excess h2so4, 0.0891 g of baso4 formed. what percentage of barium is in the compound?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Which formula can be used to calculate the molar mass of hydrogen peroxide
Answers: 1

Chemistry, 22.06.2019 12:00
What is the subscript for oxygen in its molecular formula
Answers: 1

Chemistry, 22.06.2019 14:30
Connect the whole numbers on the periodic table to indicate what they represent?
Answers: 3

Chemistry, 22.06.2019 21:00
What is the chemical formula for the compound formed between sodium and flour one
Answers: 1
You know the right answer?
Barium can be analyzed by precipitating it as baso4 and determining the mass of the precipitate. whe...
Questions

Business, 30.03.2021 22:10

Mathematics, 30.03.2021 22:10



Mathematics, 30.03.2021 22:10

History, 30.03.2021 22:10



History, 30.03.2021 22:10

Mathematics, 30.03.2021 22:10

Computers and Technology, 30.03.2021 22:10




Social Studies, 30.03.2021 22:10

Mathematics, 30.03.2021 22:10


Mathematics, 30.03.2021 22:10


Social Studies, 30.03.2021 22:10

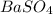 , 1 molecule of
, 1 molecule of 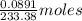 of
of 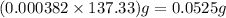
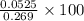 % = 19.5%
% = 19.5%

