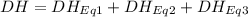At room temperature, the following heats of reaction are known: eq. 1: no(g) + o3(g) → no2(g) + o2(g) δh = –198.9 kj eq. 2: 2 o3(g) → 3 o2(g) δh = –284.6 kj eq. 3: 2 o2(g) → 4 o(g) δh = –990.0 kj use the above data to calculate the heat absorbed (kj) when 2.5 moles of no2(g) is formed at room temperature according to the chemical reaction. do not include units. no(g) + o(g) → no2(g)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Based on the law of conservation of energy, which statement is false? answer- energy is lost when machines dont work right
Answers: 1

Chemistry, 22.06.2019 03:10
The covalent compound acetylene, which is the fuel of the oxyacetylene torch used by welders, has the molecular formula c2h2. the covalent compound benzene, a commercial solvent, has the molecular formula c6h6 each of these covalent compounds contains carbon and hydrogen atoms in a one-to-one ratio. would it be correct to write the chemical formulas of each as ch? explain.
Answers: 1

Chemistry, 22.06.2019 05:00
Choose all the answers that apply. ionic compounds dissolve easily in water do not dissolve in water have low melting points have high melting points conduct electricity when melted
Answers: 1

Chemistry, 22.06.2019 05:20
Asolution contains 180 g of glucose (c6h12o6) and 162 g of water. what is the mole fraction of glucose?
Answers: 3
You know the right answer?
At room temperature, the following heats of reaction are known: eq. 1: no(g) + o3(g) → no2(g) + o2...
Questions


Computers and Technology, 15.07.2021 20:20


Mathematics, 15.07.2021 20:20

Mathematics, 15.07.2021 20:20


Mathematics, 15.07.2021 20:20

Mathematics, 15.07.2021 20:20

Mathematics, 15.07.2021 20:20




Mathematics, 15.07.2021 20:20

Social Studies, 15.07.2021 20:20

Computers and Technology, 15.07.2021 20:20

Mathematics, 15.07.2021 20:20

History, 15.07.2021 20:20

Mathematics, 15.07.2021 20:20

Mathematics, 15.07.2021 20:20

Chemistry, 15.07.2021 20:20