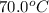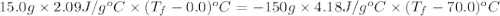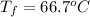15.0 g of ice cubes at 0.0°c are combined with 150. g of liquid water at 70.0°c in a coffee cup calorimeter. calculate the final temperature reached, assuming no heat loss or gain from the surroundings. (the specific heat capacity of h2o(l), cs = 4.18 j/gc; h2o(s) h2o(l) h = 6.02 kj/mol)

Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 01:30
Aroller coaster car is traveling down a track at 22 m/s. the car has a mass of 2000 kg. what is the kinetic energy of the car? a) 22,000 j b) 968,000 j c) 484,000 j d) 44,000 j
Answers: 2

You know the right answer?
15.0 g of ice cubes at 0.0°c are combined with 150. g of liquid water at 70.0°c in a coffee cup calo...
Questions



Advanced Placement (AP), 27.09.2020 01:01




Health, 27.09.2020 01:01




History, 27.09.2020 01:01




Computers and Technology, 27.09.2020 01:01


English, 27.09.2020 01:01



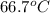
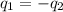
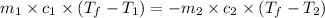
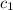 = specific heat of ice =
= specific heat of ice = 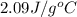
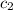 = specific heat of water =
= specific heat of water = 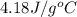
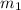 = mass of ice = 15.0 g
= mass of ice = 15.0 g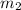 = mass of water = 150 g
= mass of water = 150 g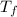 = final temperature = ?
= final temperature = ?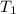 = initial temperature of ice =
= initial temperature of ice = 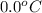
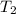 = initial temperature of water =
= initial temperature of water =