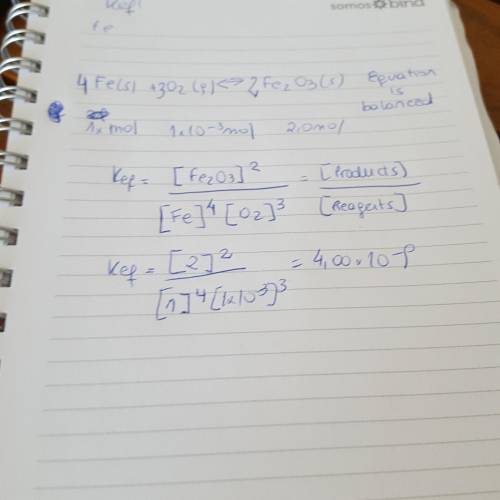Chemistry, 31.10.2019 06:31 bryanmcmillianjr
Write the equilibrium expression, calculate keq and then tell where the equilibrium lies: fe (s) + o2 (g) ↔ fe2o3 (s) in a 2.0 l container at equilibrium: fe = 1.0 mol o2 = 1.0 e-3 mol fe2o3 = 2.0 mol

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the molecular formula for a compound that is 46.16% carbon, 5.16% hydrogen, and 48.68% fluorine? the molar mass of the compound is 156.12 g/mol
Answers: 2


Chemistry, 22.06.2019 21:00
How many neutrons does an element have if its atomic number is 50 and its mass number is 166
Answers: 1

Chemistry, 22.06.2019 21:50
28. which is not a reason that water is used to store spent fuel rods from nuclear power plants? water increases the speed of the chain reaction in the fuel rods. water protects nuclear power plant workers from the high temperature and radiation of the fuel rods. water acts as a radiation shield to reduce the radiation levels. water cools the spent rods. salts action
Answers: 1
You know the right answer?
Write the equilibrium expression, calculate keq and then tell where the equilibrium lies: fe (s) +...
Questions




Mathematics, 18.11.2020 20:50

Mathematics, 18.11.2020 20:50

History, 18.11.2020 20:50


Mathematics, 18.11.2020 20:50


Social Studies, 18.11.2020 20:50

English, 18.11.2020 20:50

Social Studies, 18.11.2020 20:50


Social Studies, 18.11.2020 20:50

History, 18.11.2020 20:50

Mathematics, 18.11.2020 20:50

Mathematics, 18.11.2020 20:50

History, 18.11.2020 20:50

Mathematics, 18.11.2020 20:50

English, 18.11.2020 20:50