
Chemistry, 31.10.2019 01:31 CaraRose1887
Consider the reaction: 2 so2(g)+o2(g)→2 so3(g) if 285.5 ml of so2 reacts with 158.9 ml of o2 (both measured at 315 k and 50.0 mmhg), what is the limiting reactant and the theoretical yield of so3? if 187.2 ml of so3 is collected (measured at 315 k and 50.0 mmhg), what is the percent yield for the reaction?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 19:30
What is the common name for the compound shown here? enter the common name of the compound shown?
Answers: 2

Chemistry, 22.06.2019 21:00
How many neutrons does an element have if its atomic number is 50 and its mass number is 166
Answers: 1

Chemistry, 23.06.2019 13:20
Aluminum reacts with sulfuric acid to produce aluminum sulfate and hydrogen gas. how many grams of aluminum sulfate would be formed if 250 g h 2 so 4 completely reacted with aluminum? 2al( s ) + 3h 2 so 4 ( aq ) ? al 2 (so 4 ) 3 ( aq ) + 3h 2 ( g )
Answers: 1

Chemistry, 23.06.2019 15:30
K12 chemistry unit assessment: chemical bonding, electrostatic forces in ionic bonds hold which of the following together? 1. he atoms in helium gas 2.na+ and br- in nabr 3. fe atoms and localized electrons in iron
Answers: 1
You know the right answer?
Consider the reaction: 2 so2(g)+o2(g)→2 so3(g) if 285.5 ml of so2 reacts with 158.9 ml of o2 (both...
Questions

Physics, 19.12.2021 02:40

Physics, 19.12.2021 02:40


Mathematics, 19.12.2021 02:40



Computers and Technology, 19.12.2021 02:40

Mathematics, 19.12.2021 02:40




Health, 19.12.2021 02:40

Advanced Placement (AP), 19.12.2021 02:40



Mathematics, 19.12.2021 02:50


Mathematics, 19.12.2021 02:50


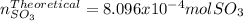
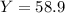 %
%
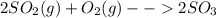
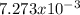 moles of
moles of 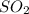 as follows:
as follows: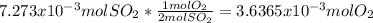
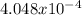 moles are available in comparison with the
moles are available in comparison with the 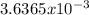 moles that completely would react with
moles that completely would react with 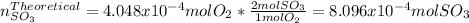
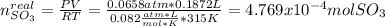
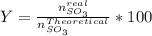 %
%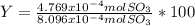 %
%

