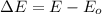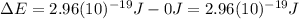Chemistry, 30.10.2019 22:31 swaggernas
A) a red laser pointer emits light with a wavelength of 670 nm. what is the frequency of this light?
b) what is the energy of 1 mole of these photons?
c) the laser pointer emits light because electrons in the material are excited (by a battery) from their ground state to an upper excited state. when the electrons return to the ground state they lose the excess energy in the form of 670 nm photons. what is the energy gap between the ground state and excited state in the laser material?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Aside from human impact, which of the following causes less water vapor production over a small area? (2 pderivartin
Answers: 1

Chemistry, 22.06.2019 17:10
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2

Chemistry, 22.06.2019 21:00
Use the measurements in the table to determine which unidentified metal has the highest density. metal volume mass a 10.5 cm3 122 g b 14.2 cm3 132 g c 16.1 cm3 115 g d 12.7 cm3 126 g
Answers: 2

Chemistry, 22.06.2019 22:00
The diagrams to the right show the distribution and arrangement of gas particles in two different containers. according to kinetic-molecular theory, which of the following statements is true? check all that apply. if the temperatures of both containers are equal, container a has greater pressure than container b. if the volume of container a decreased, its pressure would decrease. if the pressure in both containers is equal, container a has a lower temperature than container b.
Answers: 2
You know the right answer?
A) a red laser pointer emits light with a wavelength of 670 nm. what is the frequency of this light?...
Questions

Social Studies, 01.12.2021 03:20


Chemistry, 01.12.2021 03:20

Chemistry, 01.12.2021 03:20


Computers and Technology, 01.12.2021 03:20

Chemistry, 01.12.2021 03:20

English, 01.12.2021 03:20

Chemistry, 01.12.2021 03:20





Computers and Technology, 01.12.2021 03:20

Business, 01.12.2021 03:20



Mathematics, 01.12.2021 03:20


Mathematics, 01.12.2021 03:20

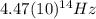
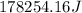
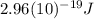
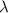 and the frequency
and the frequency  of the light:
of the light:
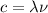 (1)
(1)
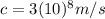 is the speed of light in vacuum
is the speed of light in vacuum
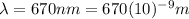 is the wavelength of the light emitted by the laser pointer
is the wavelength of the light emitted by the laser pointer
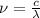 (2)
(2)
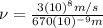 (3)
(3)
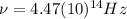 (4) This is the frequency
(4) This is the frequency
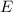 of a 670 nm photon is given by:
of a 670 nm photon is given by:
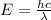 (5)
(5)
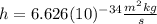 is the Planck constant
is the Planck constant 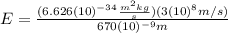 (6)
(6)
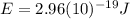 (7) This is the energy of one photon
(7) This is the energy of one photon
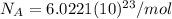 ):
):
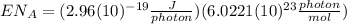 (8)
(8)
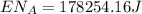 This is the energy of 1 mole of 670 nm photons
This is the energy of 1 mole of 670 nm photons
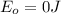 and the energy gap
and the energy gap 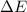 is:
is: