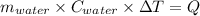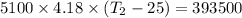Chemistry, 30.10.2019 05:31 mashedpotatoes28
One mole of carbon (12.0 g) in the form of crystalline graphite is burned at 25◦c and 1.000 atm pressure to form co2(g). all of the heat produced is used to heat a 5100 g bath of liquid water, originally at 25◦c. what is the final temperature of the water bath? the heat of formation of co2(g) is −393.5 kj/mol and the specific heat of water is 4.18 j/g/◦c. answer in units of ◦c

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
During chemistry class, carl performed several lab tests on two white solids. the results of three tests are seen in the data table. based on this data, carl has concluded that substance b must have bonds.
Answers: 2

Chemistry, 21.06.2019 22:30
Determine the wavelength of the light absorbed when an electron in a hydrogen atom makes a transition from an orbital in the n=3 level to an orbital in the n=7 level.
Answers: 2

Chemistry, 22.06.2019 04:30
Acamcorder has a power rating of 17 watts. if the output voltage from its battery is 7 volts, what current does it use?units:
Answers: 1

Chemistry, 22.06.2019 10:00
Miner's coal distributors does not mine coal itself, nor does it even store or handle the coal. instead, miner's solicits orders for low sulfur coal from other firms, then purchases the required amount from suppliers and directs them to ship the coal to its customers. what is miner's
Answers: 1
You know the right answer?
One mole of carbon (12.0 g) in the form of crystalline graphite is burned at 25◦c and 1.000 atm pres...
Questions




English, 14.02.2020 22:23




Spanish, 14.02.2020 22:23




Mathematics, 14.02.2020 22:23



Mathematics, 14.02.2020 22:23

Business, 14.02.2020 22:23