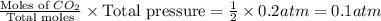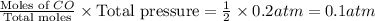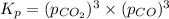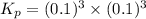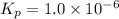When heated, lanthanum(iii) oxalate decomposes as follows: la2(c2o4)3(s) < ===> la2o3(s) + 3 co2(g) + 3 co(g) starting with just the oxalate in a 10.0 l flask, at equilibrium the total pressure observed is 0.200 atm. what is the value of kp for the equilibrium? (dalton’s law of partial pressure! )

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Calculate the expected ph values of the buffer systems from the experiments (a,b,c,d), using the henderson- hasselbalch equation, ph-pka+log[a-]/[ha]. use for pka values carbonic acid= 6.37, and acetic acid= 4.75.
Answers: 2

Chemistry, 22.06.2019 12:00
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1

Chemistry, 23.06.2019 00:00
Which is true about metals used for jewelry, such as platinum and gold? a. they have low flammability. b. they have low reactivity. c. they have high flammability. d. they have high reactivity.
Answers: 1

Chemistry, 23.06.2019 02:30
Apound is approximately 0.45 kilogram. a persons weighs 87 kilograms. what is the persons’s weight, in pounds, when expressed to the correct number of significant figures
Answers: 1
You know the right answer?
When heated, lanthanum(iii) oxalate decomposes as follows: la2(c2o4)3(s) < ===> la2o3(s) + 3...
Questions

Mathematics, 09.11.2020 17:30


Mathematics, 09.11.2020 17:30

Mathematics, 09.11.2020 17:30


English, 09.11.2020 17:30

Mathematics, 09.11.2020 17:30

Biology, 09.11.2020 17:30

Biology, 09.11.2020 17:30


Mathematics, 09.11.2020 17:30


Mathematics, 09.11.2020 17:30



Mathematics, 09.11.2020 17:30



Mathematics, 09.11.2020 17:30

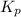 for the equilibrium is
for the equilibrium is 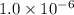
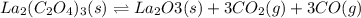
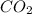 and
and 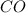 .
.